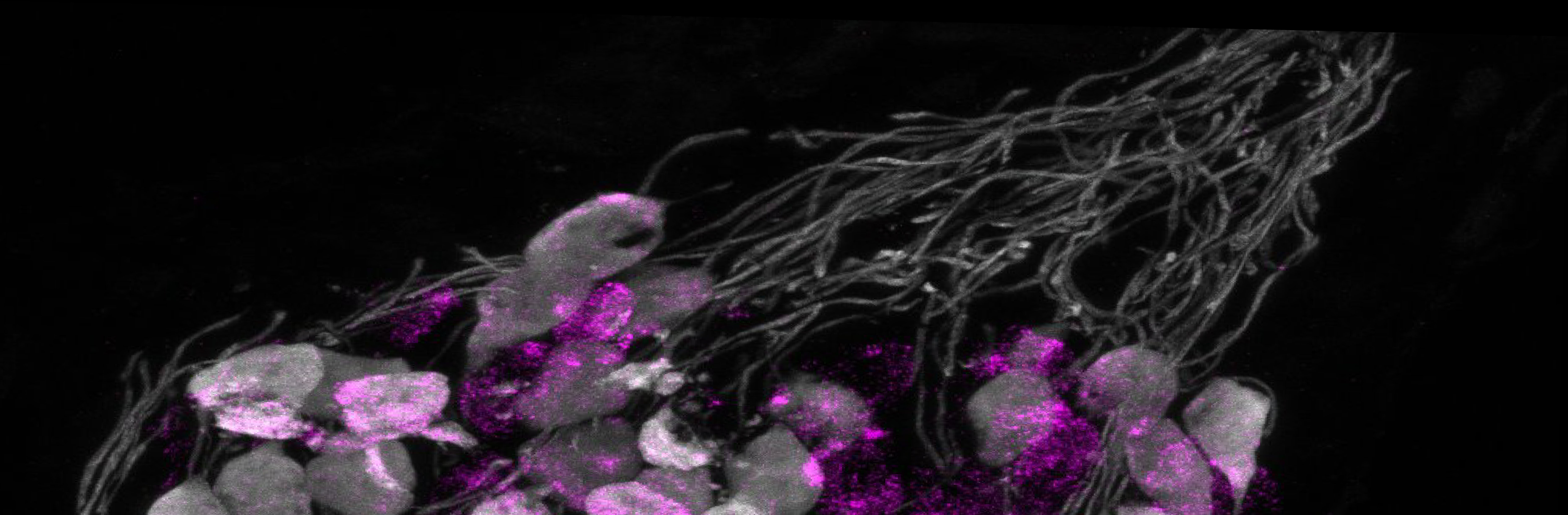
We are a neuroscience lab at Mass Eye & Ear / Harvard Medical School studying the molecular genetics of auditory sensation and perception.
 Demands for sensory coding can vary tremendously based on context and across diverse timescales
Demands for sensory coding can vary tremendously based on context and across diverse timescales
The world we inhabit as sentient beings is staggeringly complex and dynamic. Faced with the vast sensory space enclosed therein, how is our nervous system specialized to capture the slice most pertinent to us as a species? How do sensory systems adapt to reliably detect cues even as contexts fluctuate rapidly, or to meet ever-changing sensation needs over an organism’s life? Within the conceptual framework of these broad questions, our research program seeks to elucidate cellular and circuit-level features underlying faithful neural representation of ethologically relevant stimuli. To that end, we combine an array of modern molecular genetic tools with functional and behavioral assays to study relevant neural motifs in the mouse auditory system.
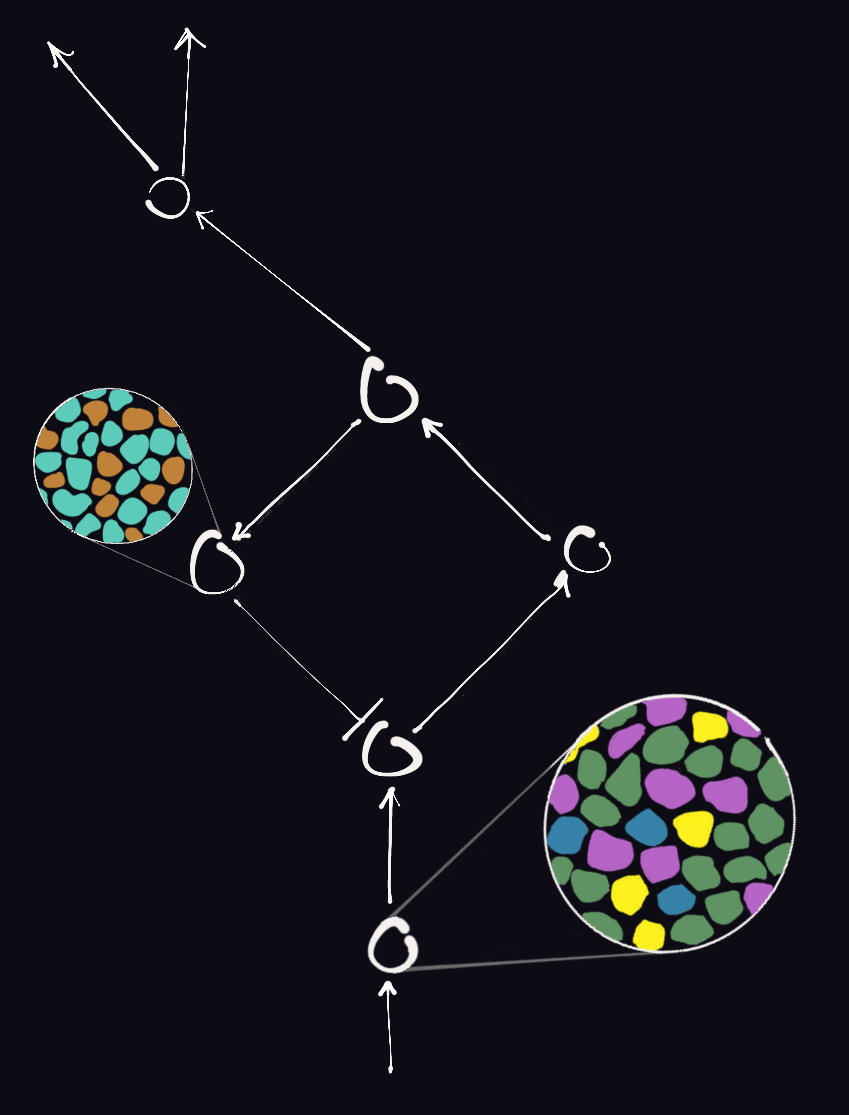 A major circuit feature of interest for the lab is cell type heterogeneity in the nervous system. Neuroscientists have been fascinated by it for eons and continue to chart cell types and connectivity maps at an unprecedented scale using modern technologies. In our pursuits, we deliberately look beyond broad subdivisions such as excitatory vs inhibitory, motor vs sensory, efferent vs afferent, and instead ask how diversity of closely-related neuronal types—those comprising a circuit node and often lumped into a singular class in coarse neural classification schemes—emerge developmentally and the computational benefit it confers to neural circuits. Recent single-cell transcriptomic studies confirm classical physiology-based insights that the nervous system is abound with this form of heterogeneity. We find sensory neurons particularly attractive in this regard for the opportunities offered to map discrete dimensions of neuronal feature variability onto qualitative or quantitative aspects of physical stimuli.
A major circuit feature of interest for the lab is cell type heterogeneity in the nervous system. Neuroscientists have been fascinated by it for eons and continue to chart cell types and connectivity maps at an unprecedented scale using modern technologies. In our pursuits, we deliberately look beyond broad subdivisions such as excitatory vs inhibitory, motor vs sensory, efferent vs afferent, and instead ask how diversity of closely-related neuronal types—those comprising a circuit node and often lumped into a singular class in coarse neural classification schemes—emerge developmentally and the computational benefit it confers to neural circuits. Recent single-cell transcriptomic studies confirm classical physiology-based insights that the nervous system is abound with this form of heterogeneity. We find sensory neurons particularly attractive in this regard for the opportunities offered to map discrete dimensions of neuronal feature variability onto qualitative or quantitative aspects of physical stimuli.
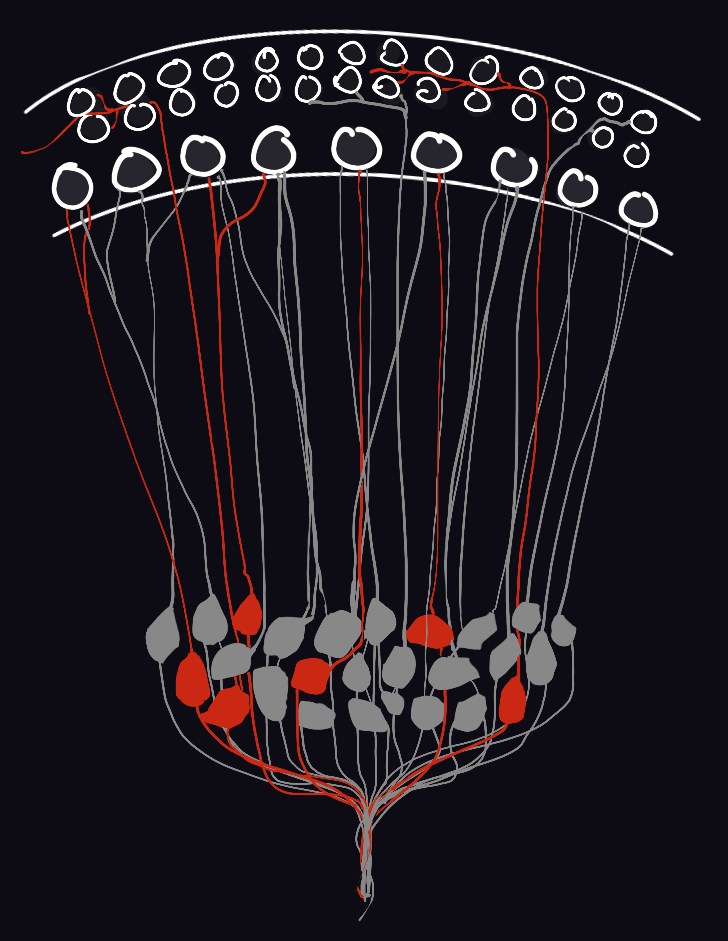
For this reason, our efforts are currently anchored on Type I spiral ganglion neurons (SGNs), the primary sensory neurons of the auditory system. There are 3 molecular subtypes of Type I SGNs; little is known about how their identities are established during development. SGN diversity is thought to be crucial for sound intensity coding and the ability to hear in noisy environments, but direct experimental evidence has remained elusive due in part to the inability to target SGN subgroups precisely. In our laboratory, we study molecular genetic mechanisms underlying diversity in the connectivity and physiological repertoires of SGNs, stability of their subtype-specific features over time, and significance of SGN subtype heterogeneity for sound sensation—and ultimately for perception. In doing so, we (1) generate and employ tools for spatiotemporally precise genetic access of SGNs; (2) read and/or perturb their activity patterns; (3) chart their transcriptomic and gene regulatory landscapes by leveraging machine learning methods; and (4) measure changes in sound perception using operant-based behavioral assays. We accomplish much of these using animal models in which gene expression is altered and neurons are molecularly tagged using adeno-associated viruses or CRISPR/Cas9 technology.
Across our scientific pursuits we strive to traverse multiple scales of inquiry, not only for tools and inspiration but also to generate integrated insights. We have a long-term interest in placing our studies in an evolutionary framework, asking how the developmental ‘blueprint’ encoded in the mouse genome may be implemented variably to produce ethologically suitable sensory abilities across species.
Our research is supported by: